Search our website:
Move business-critical pharmaceutical equipment with total confidence
For more than 30 years, we’ve been working with complex biotechnology, life sciences, medical and pharmaceutical equipment in laboratories and cleanrooms worldwide.
Our all-in-one, start-to-finish service covers every step of your equipment relocation, from single production lines to full-site relocations.






Comprehensive site surveys, method statements and risk assessments
Decontamination
Dismantling and de-installation
Equipment move-out
Export packing
Door-to-door freighting, including customs
Equipment move-in
Position, levelling and alignment


Pedestal floors
Upgrades
Compliance testing - including UKCA/CE Marking and PUWER assessments
We’ve been helping world-renowned pharmaceutical companies to move business-critical capital equipment since 1991.
How we helped Catalent move and install 18 tonnes of highly delicate critical equipment into its new laboratory.
How we helped Pirbright Institute to execute a first-of-its-kind laboratory equipment move alongside delicate livestock virus samples.
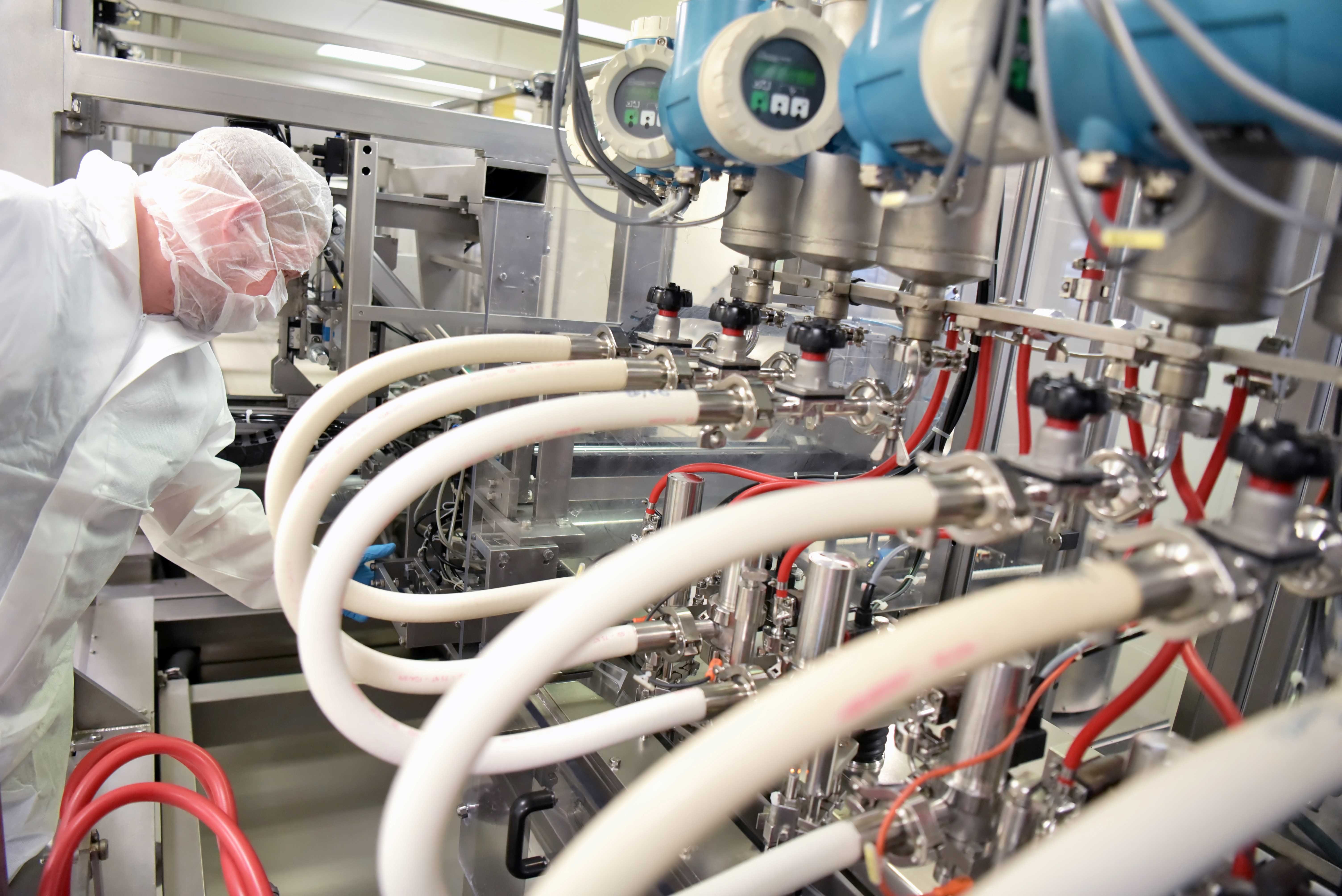

30 years of experience working with high-technology pharmaceutical equipment
Industry experts who understand equipment complexities and cleanroom protocols
A start-to-finish, end-to-end relocation service that you can rely on
Flexible teams that understand plans can change and will adapt to any new requirements
We have close relationships with leading OEMs and end users and decades of experience working with complex equipment:
IES, 1 Portview Road, Avonmouth,
Bristol BS11 9LS, UK
Email: info@ies.co.uk
Contact Us